A Researcher Is Comparing The Size Of Sarcomeres In Mice To Those In Elephants. What Will He Find?
Introduction
Sarcomeres are the basic force producing units of muscles. Under a light microscope, musculus fibers have alternate blackness and white bands due to the contractile filaments myosin (anisotropic band, A-band) and actin (isotropic band, I-band) that make up sarcomeres. Sarcomere patterns are useful indicators of myofibril integrity and have been used in diagnosing muscle pathologies (Plotnikov et al., 2008; Ralston et al., 2008). In addition, the amount of force and power a muscle can generate depends on the sarcomere lengths (Gordon et al., 1966; Lutz and Rome, 1994; Burkholder and Lieber, 1998).
Sarcomere lengths have been a crucial consequence measure for understanding and explaining basic muscle properties(Huxley and Peachey, 1961; Loma, 1953; Morgan, 1994) and musculus office inside the constraints of beast bodies (Cutts, 1988; Lutz and Rome, 1994). Sarcomere length tin be measured readily from muscle micrographs generated by low-cal microscopy (Huxley and Peachey, 1961; Telley et al., 2006; Infantolino et al., 2010) and electron microscopy (Goulding et al., 1997), or from the diffraction pattern resulting from shining a light amplification by stimulated emission of radiation beam through a muscle (ter Keurs et al., 1978). For lite or electron microscopy, muscle cells need to be isolated through mechanical or enzymatic ways (Huxley and Peachey, 1961; Goulding et al., 1997; Telley et al., 2006; Infantolino et al., 2010). Although, laser diffraction allows measurement of sarcomere length in vivo (Takahashi et al., 2007), a pocket-sized muscle fascicle must exist locally dissociated from the whole musculus for total penetration of the light amplification by stimulated emission of radiation low-cal. Equally a upshot, all of the same approaches involve a high degree of invasiveness. Contempo advances in non-linear microscopy make visualization of sarcomeres in living muscles possible through 2d harmonic generation (SHG) imaging (Campagnola and Loew, 2003; Plotnikov et al., 2006). Sarcomeres were successfully imaged in vivo using a micrometer-sized endoscope (Llewellyn et al., 2008; Cromie et al., 2013). Nevertheless, this arroyo requires the insertion of the micro-endoscope into the muscle and it remains unclear what result such a mechanical perturbation might have on the resulting sarcomere length. Therefore, an comeback to existing techniques involves the examination of sarcomere lengths that involves no contact with the musculus.
Sarcomere length for a given muscle is typically measured at a single spot, often in the mid-abdomen of the muscle, and at a given musculus length (east.g., Cutts, 1988). Information technology is then causeless implicitly that the sarcomere length measured at this unmarried spot represents the sarcomere lengths at other locations within the muscle, and force-length, force-velocity, and power-velocity backdrop of muscles are oft implied based on these single sarcomere length measurements (Rack and Westbury, 1969; Lutz and Rome, 1994; Burkholder and Lieber, 2001; Vaz et al., 2012). Notwithstanding, information technology has been shown in single fibers (Huxley and Peachey, 1961; Infantolino et al., 2010), and whole muscles (Llewellyn et al., 2008) that sarcomere lengths tin can be, and typically are quite variable. However, sarcomere length variability within a musculus has not been systematically analyzed. Furthermore, it has been tacitly assumed that when muscles alter length, all fibers in the muscle, independent of their initial length, change length in such a manner that sarcomere lengths remain uniform across the entire muscle (Cutts, 1988), an intuitively highly-seasoned assumption, but one that has no systematic scientific support.
Therefore, the purpose of this written report was to measure sarcomere lengths at defined locations along and across an intact muscle, at unlike muscle lengths. Sarcomere length variations were quantified among approximately 240–320 sarcomeres at a given location, for v different locations within the musculus, and three dissimilar lengths encompassing the anatomical range of movement of the mouse tibialis anterior (TA). All measurements were performed in passive muscle. We hypothesized that sarcomere lengths differ locally, every bit has been shown in unmarried fibers and entire muscles before. In addition, based on the implicit assumption used in previous studies, we hypothesized that mean sarcomere lengths were similar at different locations of the muscle and that sarcomere length changes were also uniform across the dissimilar locations.
Materials and Methods
Animal Preparation
All aspect of animate being care and experimental procedures were carried out in accordance with the guidelines of the Canadian Quango on Creature Care and were approved past the Academy of Calgary Life Sciences Animal Enquiry and Ethics Committee. X to twelve calendar week-old male person C57BL6 mice (north = 5) were anesthetized using a 1–3% isoflurane/oxygen mixture that was delivered through mask ventilation at a period rate of 0.6 l/min. The state of anesthesia was monitored through foot compression of the contralateral limb. The left hind limb was skinned. The fascia over the tibialis anterior (TA) muscle was removed. Then, the left genu of the mouse was stock-still by a custom-made clamp, while the left pes was pinned to a movable base that immune adjustment of the ankle angle. The Achilles tendon of the left hind limb was cut to allow for a full range of motion of the ankle without resistance from the triceps surae muscles. Throughout the experiment, the left TA musculus was immersed in a phosphate buffered saline (P5368, Sigma Aldrich, Ontario, Canada) with a pH of 7.iv in a custom-made h2o sleeping room and was kept at room temperature (~21°C) to go along the muscle hydrated and to allow for water-immersion imaging. Later the imaging protocols, the mice were sacrificed by a barbiturate overdose using 0.5 ml Euthanyl (pentobarbital sodium, Biomeda-MTC pharmaceuticals, Cambridge, Ontario, Canada).
In vivo Imaging of Muscle
Two reference points were defined at the proximal and distal ends of the TA (Figure 1). From these reference points, five landmarks along the longitudinal centrality of the muscle ("proximal," "middle," "distal" landmarks) and along the transverse axis of the muscle ("medial," "middle," "lateral" landmarks) were defined. The "middle" landmark was the mid-point of the straight line connecting the "proximal" and "distal" landmarks, while the "medial" and "lateral" landmarks were ~500 μm away from the "middle" landmark, respectively.
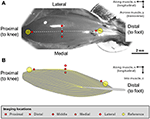
Figure 1. (A) Digital photo of the mouse tibialis anterior (TA) muscle. (B) Schematic analogy of the muscle architecture in the mid-sagittal airplane of the musculus (Heemskerk et al., 2005; Lovering et al., 2013). The fiber orientation is indicated by the yellow outlines. The sarcomere lengths of the mouse tibialis anterior musculus were imaged at 5 defined sites. Ii reference points were offset defined at the proximal and distal ends of the TA musculus. From these reference points, 5 imaging sites located along the longitudinal axis ("proximal," "middle," "distal" sites) and forth the transverse axis ("medial," "middle," "lateral" sites) were identified on TA. The "middle" landmark was the mid-point of the direct line connecting the "proximal" and "distal" TA sites, while the "medial" and "lateral" landmarks were ~500 μm away from the "centre" landmark in the medial and lateral directions, respectively.
Second harmonic generation (SHG) imaging of the TA was performed using an upright, multi-photon excitation microscope (FVMPE-RS model, Olympus, Tokyo, Japan) equipped with a wavelength-tunable (680–1300 nm), ultrashort-pulsed (pulse width: < 120 fs; repetition rate: fourscore MHz) light amplification by stimulated emission of radiation (InSight DeepSee-OL, Spectra-Physics, CA, U.s.) and a 25 x/1.05 NA water immersion objective (XLPLN25XWMP2 model, Olympus, Tokyo, Japan). The TA was scanned using a laser wavelength of 800 nm. The resulting SHG indicate emitted by the musculus was collected in the backward (epi-) direction using a ring-pass filter at the harmonic frequency (FF01 400/40, Semrock Inc., NY, USA). The average power in the sample plane was varied from 14 to forty mW in guild to produce optimal images.
At each landmark, a series of planar images were acquired in the horizontal plane (xy-plane; imaging area: 159 × 159 μm; pixel size: 0.155 μm; bit-depth: 12; dwell time: 2 μs) forth the objective axis (z-axis) that is perpendicular to the horizontal plane at i μm intervals. Image stacks were taken from the meridian thirty μm of the musculus (denoted as the surface zone) at all v imaging landmarks. For imaging landmarks that were located forth the transverse axis of the musculus ("medial," "middle," and "lateral"), additional thirty μm thick image stacks were acquired from tissue that was 100 μm deep (denoted every bit the deep zone). The SHG imaging of the muscle was repeated at 3 ankle angles respective to full dorsiflexion (angle between pes and tibia ~50°), intermediate angle (~120°), and total plantarflexion (~180°). Just musculus images costless of cardiac and respiratory motion artifacts were included in the image analysis.
Epitome Assay
Due to the spindle-like shape of the mouse TA, paradigm stacks were sequentially rotated in the xy- and xz-plane using "TransformJ: Rotate" plugin of ImageJ (National Establish of Wellness, MD, USA) in club to align the longitudinal centrality of the musculus then that the epimysium was always parallel to the horizontal axis for ease of comparisons beyond the different landmarks (run into Supplementary Materials, Figure S6).
From the rotated image stacks, iv representative planar image bands of 30 pixels (~4.7 μm) wide that had expert signal-to-noise ratio and independent 15–twenty sarcomeres in serial were selected from each of 4 sub-regions per site. By bold a myofibril diameter of ~one.three μm (Powers et al., 2016), we could decide that each planar prototype band contained lx–80 sarcomeres (~iv sarcomeres in parallel containing ~15–xx sarcomeres in series). And so, each image band was filtered by a patch-based de-noising algorithm (Chatterjee and Milanfar, 2012) using fifty patches of v-pixel radius and an iteration number of xx. The resulting image band was farther analyzed past a custom written MATLAB code that identified the centroids of the sarcomeric A-bands. Private sarcomere lengths were measured as the distance between adjacent A-band centroids (Figure ii).
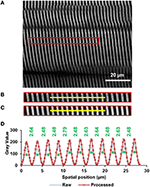
Figure 2. Sarcomere lengths were measured from images obtained by 2nd harmonic generation (SHG). The A-bands appear as white bands in the image. The image was taken from the middle site of the TA muscle of mouse #4 (see "Supplementary Materials," Tabular array S1) during full plantarflexion of the ankle joint. (A) A representative region of interest of 30 pixels wide that contains 20 sarcomeres (indicated by the red rectangle) was selected from the images (see text in Methods for details), (B) raw paradigm, and (C) filtered prototype of the selected region. The epitome was filtered by a patch-based de-noising algorithm (Chatterjee and Milanfar, 2012), (D) Intensity profiles across the xanthous line highlighted in the raw (dotted blue line) and filtered (rectangle symbols on solid red line) muscle paradigm. The centroids of all A-bands (green asterisks) were identified using a custom-written MATLAB code. Individual sarcomere lengths were calculated as the altitude (in micrometer) between adjacent A-band centroids.
Consequence Measures
We quantified iv primary outcome measures: Start, we determined the mean sarcomere lengths from the 240–320 sarcomere lengths measured at each location (distal, heart, proximal, medial, and lateral) in a muscle and this was done for surface lying sarcomeres at each site. For the deep lying sarcomeres, the mean sarcomere lengths were only measured at the medial, middle, and lateral sites. Second, we measured the mean sarcomere elongations associated with muscle lengthening from the fully dorsi-flexed to the fully plantar-flexed position at all muscle sites. Third, we adamant the variations in sarcomere lengths at each site and expressed them as local standard deviations (SD) and local coefficients of variation (CV = standard departure/mean); and fourth, we determined the variations in sarcomere lengths beyond the entire TA and expressed them equally global SD and global CV. These final variations in sarcomere lengths were determined forth the TA (using the proximal, middle, and distal site values) and across the TA (using the medial, centre and lateral sites).
Statistical Analysis
Descriptive statistical assay was performed on the data of all muscles (n = five), by calculating means ± 1 standard deviations (SD) of sarcomere lengths at each muscle site (local analysis) and averaged across all sites of the TA (global analysis). Means of hateful sarcomere length, sarcomere elongation, local SD, local CV, global SD, and global CV, were compared between the different sites on the muscle and for the three unlike musculus lengths using two-fashion repeated measures ANOVA (with Bonferroni aligning). In addition, means of mean sarcomere lengths were also compared between the surface zone sarcomeres and the deep zone sarcomeres and for the 3 muscle lengths using 2-way repeated measures ANOVA (with Bonferroni adjustment) with α = 0.05 (SPSS 21, SPSS Inc., IL, USA).
Results
The average mass of the mice was 26.three ± 2.three g. Surface zone and deep zone sarcomeres had similar boilerplate lengths (data non shown). Unless specified otherwise, the results presented in the following paragraphs are from the surface zone sarcomeres exclusively.
Average sarcomere lengths varied substantially for the different TA sites. At full dorsiflexion (shortest muscle length), mean sarcomere lengths ranged from 2.i to 2.3 μm, with sarcomeres at the "medial" site being significantly longer than sarcomeres at the "proximal" site (Figure 3). When the TA was stretched passively by moving the talocrural joint from full dorsiflexion to full plantarflexion, sarcomeres at the different TA sites were elongated between ten and 25% of their original length. Going from the fully dorsi-flexed to the intermediate ankle position, only the sarcomere lengths at the "distal," "centre," and "lateral" sites were elongated significantly while those at the proximal and medial sites elongated to a smaller and not-significant degree (Figures 3A,B).
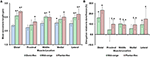
Figure three. (A) Mean lengths of surface zone sarcomeres at the five target sites ("distal," "proximal," "centre," "medial," and "lateral") of the tibialis anterior (TA) muscle at full dorsiflexion, intermediate ankle angle, and full plantarflexion. (B) Elongation of sarcomeres relative to the sarcomere lengths at full dorsiflexion. The graphs testify the average values of the mean sarcomere lengths (A) and elongations (B) for the 5 mice. Sarcomere lengths varied with locations on the TA. When the TA was lengthened, sarcomeres at all sites elongated but in a location-dependent manner. Sarcomeres at the "proximal" TA site were shorter compared to sarcomeres at the other sites. When the TA was elongated, sarcomeres at the "distal" TA site lengthened more than sarcomeres at the other sites. *Indicates significant differences in sarcomere length compared to the sarcomeres at the proximal TA site for a item talocrural joint position (p < 0.05). aShows significant differences in sarcomere length or sarcomere elongation compared to the corresponding length at full dorsiflexion (p < 0.01). †Represents pregnant differences in sarcomere elongation compared to the sarcomeres at the distal TA site (p < 0.05; see section Statistical Analysis in the "Methods" for more details).
Sarcomere lengths varied along fibers at each TA site and also varied betwixt the 5 TA sites. Locally, sarcomere length in series had standard deviations (SD) of ~0.ane μm, and the local coefficient of variation (CV) was ~5% at all TA sites, except for the "proximal" site where sarcomeres had higher local SD (~0.xviii μm) and local CV of (~viii%) at full dorsiflexion. These variations in sarcomere lengths at the "proximal" TA site decreased as the TA was stretched from the dorsi-flexed, to the intermediate, and the full plantar-flexed talocrural joint position (Figures 4A,B). Sarcomere length variations across the different TA sites were greater along the muscle than across the muscle with global SD of ~0.20 μm (along TA) vs. ~0.12 μm (across TA) and global CV of ~viii% (along TA) vs. ~five% (across TA; Figure 5).
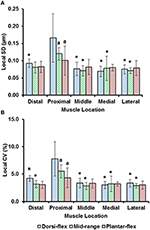
Figure four. Local dispersion of sarcomere lengths at total dorsiflexion, intermediate ankle angle, and full plantarflexion given by (A) the local standard deviation ( SD ) and, (B) the local coefficient of variation ( CV ). For each TA site of 159 × 159 μm, the local dispersion of sarcomere lengths was measured from four representative image bands that were of iv.seven μm wide and contained fifteen–twenty sarcomeres in series. The graphs show the boilerplate values of the local SDs (a) and local CVs (b) for the five mice. Sarcomeres at the "proximal" TA site had higher local SD and local CV compared to sarcomeres at the remaining TA sites. *Indicates significant differences in local SD and local CV compared to the sarcomeres at the proximal TA site and a given ankle joint bending (p < 0.05). a Indicates significant differences in local SD and local CV compared to sarcomeres in the fully dorsi-flexed position (p < 0.05; see Section Statistical analysis in the "Methods" for more details).
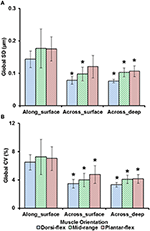
Effigy 5. Global dispersion in sarcomere lengths for surface zone and deep zone sarcomeres at full dorsiflexion, intermediate talocrural joint angle, and full plantarflexion. (A) Global standard deviation (SD) and, (B) global coefficient of variation (CV). Sarcomeres from the surface zone were pooled based on TA site, either into measurements forth the longitudinal axis ("proximal," "center," and "distal" TA sites) or along the transverse axis of the muscle ("medial," "middle," and "lateral"). In addition, sarcomeres from the deep zone that were located along the transverse centrality of the TA were analyzed for their dispersions. The graphs show the average of the global SDs and global CVs from the five mice tested. Surface zone sarcomeres along the longitudinal axis of the TA had greater dispersions of length compared to sarcomeres that were aligned along the transverse axis of the TA. *Indicates pregnant differences in global SD and global CV compared to surface zone sarcomeres that were located along the longitudinal axis of the TA at a given ankle bending (p < 0.05; see Section Statistical Analysis in the "Methods" for more details).
The mean, standard deviation, minimum value, and maximum value of sarcomere length for all five TA sites and all five muscles are summarized in Table S1 (Encounter Supplementary Material). In a region of 159 × 159 μm2, the local departure between the longest and the shortest sarcomere were as high as 1 μm (Table S1). The great range of sarcomere lengths is illustrated in the besprinkle plot of individual sarcomere lengths against the mean sarcomere lengths pooled across all muscles and all TA sites analyzed (Figure 6). Besprinkle plots of private sarcomere length confronting mean sarcomere length of the private TA sites are also shown, for abyss (Figures S1–S5; Supplementary Textile). From the pooled data, the proportion of sarcomeres on the ascending limb, plateau region, and descending limb of the theoretical strength-length curve were also quantified (Effigy 7).

Figure 6. Scatter plot of individual sarcomere lengths against hateful sarcomere lengths across all 5 TA sites where measurements were made ( n = 5). Results are shown for the ankle in a position of full dorsiflexion (blueish), intermediate bending (greenish), and full plantarflexion (blood-red). The optimal range of sarcomere lengths of mouse TA was calculated from the known lengths of the thin and thick myofilaments (Gokhin et al., 2014). The optimal length is identified by the yellowish bands (2.30–ii.47 μm). The lengths of serially arranged sarcomeres varied substantially fifty-fifty in the minor local regions. Private sarcomere length variability may exist underestimated when sarcomere lengths are expressed as mean values beyond a region, every bit evidenced by the wider range of sarcomere lengths along the y-axis (individual lengths) compared to those along the x-axis (mean lengths). All data points from all v animals (corresponds to 1200–1500 sarcomeres) are shown in each of the graphs. Come across Supplementary Materials for scatter plots of each imaging site.
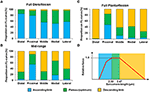
Effigy seven. Proportion of sarcomeres with lengths that fall onto the ascending limb (blue confined), plateau region (green bars) and descending limb (orangish bars) of the theoretical forcefulness-length curve at (A) total dorsiflexion, (B) intermediate ankle angle, and (C) full plantarflexion. A representative sarcomere force-length curve (red curve) of mouse TA is shown in (D). The theoretical optimal sarcomere length ranges from 2.30 to ii.47 μm (Walker and Schrodt, 1974; Gokhin et al., 2014).
Word
In the electric current study, the distribution of passive muscle sarcomere lengths over the total range of motion of intact, living whole muscle was systematically investigated using non-linear SHG imaging. With multi-photon microscopy, muscle striation patterns (I- and A-bands) tin be visualized through the interaction of the high intensity laser beam with the myosin thick filaments that are organized not-centrosymmetrically (Plotnikov et al., 2006). Since a strong SHG bespeak can be detected easily fifty-fifty at a low laser power (Llewellyn et al., 2008; Cromie et al., 2013), individual sarcomere lengths tin be measured direct from the whole muscle in vivo in a mode that does not require contact with the muscle that could impact local sarcomere lengths and sarcomere lengths not-uniformities. Mice were kept alive throughout the experiment to avoid muscle rigor (Burkholder and Lieber, 2001). The mouse TA was chosen for 3 reasons: (one) information technology provides easy access for imaging, (2) although TA muscle is a pennate muscle (Heemskerk et al., 2005; Lovering et al., 2013), its musculus fibers run by and large forth the muscle surface, which facilitates imaging of sarcomeres at any location; and (3) TA is a single-joint muscle, so its muscle tendon unit length is uniquely given by the ankle joint bending. Every bit imaging of each planar image took ~2.1 southward, any cardiac or respiratory movements during musculus scanning may cause a distortion of the planar prototype. We controlled for possible motion artifacts by deep anesthesia of the mice to deadening the animate and middle rates, by fixing the knee rigidly to a custom-made knee clamp that isolate the lower leg and TA musculus from cardiac and respiratory movements, and by carefully selecting images gratuitous of motion artifacts during image processing.
We found that sarcomere lengths varied substantially between TA sites (Figure iii). In general, sarcomeres at the "proximal" TA site were shorter than sarcomeres at the other sites (Effigy 3A). When the TA was passively stretched, the corporeality of sarcomere lengthening was location-dependent, with sarcomeres at the "distal" TA site being stretched more than (by upwardly to 25%) than sarcomeres at the other sites (Effigy 3B). The location-dependent sarcomere elongations can exist attributed to the muscle architecture of TA, with the musculus being unipennate on the proximal end merely bipennate on the distal end (Heemskerk et al., 2005). Although, the muscle length changes due to changes in ankle angle were not measured, previous studies suggest that TA muscle elongations are linearly related to changes in ankle angles (Burkholder and Lieber, 1998, Figure 4). All the same, sarcomere length elongations were not linear, with increases in sarcomere lengths existence much greater when going from the dorsi-flexed to the mid ankle position compared to when going from the mid ankle to the fully plantar-flexed position (Figure 3B). Such non-linear elongation of sarcomeres along TA muscle may be caused by the spindle-like shape of the muscle (Figure 1B). The distal muscle has a smaller cross sectional area compared to the middle and proximal muscle. Therefore, a passive stretch of the muscle may consequence in greater sarcomere elongations in the distal compared to the heart and proximal region as there are fewer sarcomeres in parallel, and therefore, the muscle may be less stiff in the distal compared to the center and proximal regions. At the "middle" TA site, sarcomeres were not stretched, on average, when the talocrural joint was moved from the intermediate angle to the fully plantar-flexed talocrural joint angle.
Previous studies typically investigated sarcomere length locally from a minor region of the musculus (Cutts, 1988; Llewellyn et al., 2008; Cromie et al., 2013). In the electric current written report, sarcomere lengths in mouse TA muscle were measured locally from a small region (159 × 159 μm2) of the musculus, and globally from distinct sites along and beyond the unabridged muscle in order to quantify the variability of sarcomere lengths. Locally, we establish that the standard difference (SD) and coefficient of variation (CV) of sarcomere lengths were ~0.1 μm and 5%, respectively (Figure iv). These values are comparable to previous in vivo studies using whole musculus (Llewellyn et al., 2008; Cromie et al., 2013), ex vivo studies using muscle biopsies (Plotnikov et al., 2008), in vitro studies using muscle fibers (Infantolino et al., 2010) and in vitro studies using isolated myofibrils (Rassier and Pavlov, 2010; Johnston et al., 2016). It should be noted that fifty-fifty though the SD and CV-values are relatively small-scale, the differences betwixt the longest and the shortest sarcomeres from these small local regions tin can be equally loftier as 1 μm, which corresponds to well-nigh 40% of the average sarcomere length (Table S1, Figure 6). When the data were pooled to include the unabridged muscle, the global dispersion of sarcomere lengths, specially along the longitudinal axis of the muscle, was higher than the local sarcomere length non-uniformities. The global SD and CV for surface zone sarcomeres located along the longitudinal axis of the TA were ~0.2 μm and ~viii%, respectively (Figure v).
Nosotros also looked into the length range over which sarcomeres operate at rest. Based on the classic cross-bridge theory, the optimal operating length of sarcomeres of the mouse TA was calculated to range betwixt 2.30 and 2.47 μm (actin filament: ane.1 μm; (Gokhin et al., 2014), myosin filament: one.6 μm, Z-disks: 0.one μm, bare zone: 0.17 μm). Although sarcomeres are thought to produce the highest force and ability at their optimal lengths (Gordon et al., 1966), sarcomeres did not operate exclusively at their optimal lengths (Figures vi, vii). At total dorsiflexion, sarcomeres of the mouse TA were constitute to be more often than not on the ascending limb and plateau region of the force-length curve. With increasing TA lengths, the sarcomeres were shifted toward the plateau region and the descending limb of the force-length curve. However, under no conditions were the sarcomeres observed to stay exclusively at their optimal lengths with the changes in TA length.
The results of this study indicate that neither local nor global sarcomere lengths are uniform in the passive mouse TA muscle. The local non-uniformities in sarcomere lengths are similar to those observed in single fibers (Huxley and Peachey, 1961; Infantolino et al., 2010), isolated myofibrils (Rassier and Pavlov, 2010; Johnston et al., 2016), and locally, also in entire muscles (Llewellyn et al., 2008; Cromie et al., 2013). This result, combined with the findings from other studies, suggests that sarcomere lengths are naturally non-uniform in muscles, and that the results observed here are likely applicative across vertebrate skeletal muscles. The range of sarcomere lengths observed locally differed by more than 1.0 μm, which is more than 40% of the optimal sarcomere length.
Novel to the literature is the fact that the mean sarcomere lengths measured at the unlike sites of the mouse TA besides differ, and that sarcomere elongations with muscle lengthening are non-uniformly distributed across the muscle. Sarcomere length changes of as picayune as x% and as much as 25% were observed when the TA was moved through its natural in vivo length range. The functional implications of these local and global sarcomere length not-uniformities remain unknown as it has not been possible to measure out sarcomere length in active muscle simultaneously at different muscle sites. Furthermore, sarcomere lengths and associated length non-uniformities take not been explored in the context of active, dynamic properties, such equally the force-length or strength-velocity properties. These questions need to be addressed in future works. Even so, it is rubber to assume that based on the findings of this written report, it seems overly simplistic to mensurate sarcomere lengths of a muscle at a single location (Cutts, 1988; Takahashi et al., 2007), and a single muscle lengths and infer sarcomere lengths for other locations on the muscle or for other muscle lengths.
There are limitations in this written report that need careful consideration when interpreting our results. Showtime, several layers of skin and fascia covering the TA were removed for optimal SHG imaging. Notwithstanding, this process did not result in visible changes to the structure of the musculus (Figure 1), thus nosotros presume that sarcomere length measurements obtained in this way are identical to those that would be obtained in the fully intact musculus. Second, due to the limitations in tissue penetration of the SHG arroyo, the deep zone sarcomere length could only be obtained from a tissue depth of upward to 130 μm.
Despite these limitations, we provide novel insight into the distribution of sarcomere lengths over an entire muscle. Time to come studies should quantify sarcomere length variability in intact whole muscles that are activated. In vitro studies with isolated myofibrils suggest that sarcomere length non-uniformity increases from the passive to the agile country (Telley et al., 2006; Rassier and Pavlov, 2010; Johnston et al., 2016). Even so, this finding might non hold for entire muscle preparations. Also, although TA is essentially a fast-twitch muscle with virtually no type I fibers (Burkholder et al., 1994; Allen et al., 2001), type II fibers can be divided into type IIa, blazon IIx, and type IIb fibers. Time to come measurements should investigate the influence of muscle cobweb type on the sarcomere length variation. Finally, future evaluation of individual sarcomere lengths should be performed for dynamic contractions of everyday movements while following a small subset of sarcomeres during these contractions. At present, such information remain elusive and much technical development will be required before nosotros can follow a set up of sarcomeres during normal every mean solar day contractions in an intact muscle continuously.
In summary, to our cognition, this is the starting time study in which passive sarcomere length variability was determined beyond an entire muscle using a non-contact approach. We showed that sarcomere lengths varied substantially within small-scale regions of the musculus and too for dissimilar sites across the muscle. Furthermore, sarcomere elongations were non-linear with muscle length and they were highly dependent on the precise location of the sarcomeres on the muscle: the highest sarcomere stretches occurring near the distal myotendinous junction. Equally a outcome, muscle mechanics derived from sarcomere length measured from a small region of a musculus may not represent well the sarcomere length and associated functional properties of the entire muscle.
Author Contributions
Substantial contributions to the conception or design of the piece of work; or the conquering, assay, or interpretation of data for the work: EM, RF, SS, ZA, WH. Drafting the work or revising it critically for important intellectual content: EM, RF, SS, ZA, WH. Final approval of the version to exist published: EM, RF, SS, ZA, WH. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accurateness or integrity of any function of the work are appropriately investigated and resolved: EM, RF, SS, ZA, WH.
Funding
This study was supported by the Alberta AI-HS Team grant on osteoarthritis (grant number: 200700596), AI-HS postdoctoral fellowship (grant number: 10013510), NSERC CREATE training program of Biomedical Engineers for the Twenty-outset Century (grant number: CREAT/371280-2009), the Canadian Institutes of Health Research (CIHR; grant number: MOP-111205 and 140824), the Canada Research Chair Programme, and the Killam Foundation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or fiscal relationships that could exist construed equally a potential disharmonize of interest.
Acknowledgments
The authors would similar to give thanks Tim Leonard for useful discussion on data estimation.
Supplementary Material
The Supplementary Fabric for this article can be institute online at: https://www.frontiersin.org/article/10.3389/fphys.2016.00187
References
Allen, D. L., Harrison, B. C., Maass, A., Bell, M. L., Byrnes, W. C., and Leinwand, Fifty. A. (2001). Cardiac and skeletal muscle adaptations to voluntary bicycle running in the mouse. J. Appl. Physiol. 90, 1900–1908.
PubMed Abstract | Google Scholar
Burkholder, T. J., Fingado, B., Baron, South., and Lieber, R. L. (1994). Relationship betwixt muscle cobweb types and sizes and muscle architectural properties in the mouse hindlimb. J. Morphol. 221, 177–190. doi: ten.1002/jmor.1052210207
PubMed Abstract | CrossRef Full Text | Google Scholar
Burkholder, T. J., and Lieber, R. L. (1998). Sarcomere number adaptation after retinaculum transection in adult mice. J. Exp. Biol. 201, 309–316.
PubMed Abstract | Google Scholar
Burkholder, T. J., and Lieber, R. Fifty. (2001). Sarcomere length operating range of vertebrate muscles during movement. J. Exp. Biol. 204, 1529–1536.
PubMed Abstruse | Google Scholar
Campagnola, P. J., and Loew, 50. M. (2003). Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 21, 1356–1360. doi: 10.1038/nbt894
PubMed Abstract | CrossRef Full Text | Google Scholar
Cromie, 1000. J., Sanchez, One thousand. N., Schnitzer, G. J., and Delp, South. L. (2013). Sarcomere lengths in human extensor carpi radialis brevis measured by microendoscopy. Muscle Nerve 48, 286–292. doi: ten.1002/mus.23760
PubMed Abstract | CrossRef Full Text | Google Scholar
Gokhin, D. S., Dubuc, E. A., Lian, Yard. Q., Peters, Fifty. Fifty., and Fowler, V. M. (2014). Alterations in thin filament length during postnatal skeletal muscle development and crumbling in mice. Front. Physiol. five:375. doi: 10.3389/fphys.2014.00375
PubMed Abstract | CrossRef Full Text | Google Scholar
Gordon, A. K., Huxley, A. F., and Julian, F. J. (1966). The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 184, 170–192. doi: 10.1113/jphysiol.1966.sp007909
PubMed Abstract | CrossRef Full Text | Google Scholar
Goulding, D., Bullard, B., and Gautel, K. (1997). A survey of in situ sarcomere extension in mouse skeletal muscle. J. Musculus Res. Jail cell Motil. 18, 465–472. doi: 10.1023/A:1018650915751
PubMed Abstruse | CrossRef Full Text | Google Scholar
Heemskerk, A. M., Strijkers, G. J., Vilanova, A., Drost, M. R., and Nicolay, Thou. (2005). Determination of mouse skeletal muscle compages using three-dimensional diffusion tensor imaging. Magn. Reson. Med. 53, 1333–1340. doi: 10.1002/mrm.20476
PubMed Abstract | CrossRef Full Text | Google Scholar
Infantolino, B. W., Ellis, M. J., and Challis, J. H. (2010). Individual sarcomere lengths in whole musculus fibers and optimal cobweb length computation. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 293, 1913–1919. doi: 10.1002/ar.21239
PubMed Abstruse | CrossRef Full Text | Google Scholar
Johnston, K., Jinha, A., and Herzog, Due west. (2016). The role of sarcomere length non-uniformities in residue strength enhancement of skeletal muscle myofibrils. R. Soc. Open Sci. 3:150657. doi: 10.1098/rsos.150657
PubMed Abstract | CrossRef Full Text | Google Scholar
Llewellyn, M. E., Barretto, R. P. J., Delp, S. L., and Schnitzer, M. J. (2008). Minimally invasive loftier-speed imaging of sarcomere contractile dynamics in mice and humans. Nature 454, 784–788. doi: ten.1038/nature07104
PubMed Abstract | CrossRef Full Text | Google Scholar
Lovering, R. Yard., Shah, S. B., Pratt, Due south. J. P., Gong, Westward., and Chen, Y. (2013). Compages of healthy and dystrophic muscles detected past optical coherence tomography. Muscle Nervus 47, 588–590. doi: ten.1002/mus.23711
PubMed Abstract | CrossRef Full Text | Google Scholar
Morgan, D. (1994). An explanation for residual increased tension in striated muscle after stretch during contraction. Exp. Physiol. 79, 831–838. doi: 10.1113/expphysiol.1994.sp003811
PubMed Abstruse | CrossRef Full Text | Google Scholar
Plotnikov, S. V., Kenny, A. G., Walsh, S. J., Zubrowski, B., Joseph, C., Scranton, Five. L., et al. (2008). Measurement of muscle disease by quantitative 2nd-harmonic generation imaging. J. Biomed. Opt. xiii, 044018. doi: 10.1117/1.2967536
PubMed Abstruse | CrossRef Full Text | Google Scholar
Plotnikov, South. V., Millard, A. C., Campagnola, P. J., and Mohler, W. A. (2006). Characterization of the myosin-based source for second-harmonic generation from musculus sarcomeres. Biophys. J. 90, 693–703. doi: x.1529/biophysj.105.071555
PubMed Abstract | CrossRef Full Text | Google Scholar
Powers, K., Nishikawa, K., Joumaa, V., and Herzog, W. (2016). Decreased strength enhancement in skeletal muscle sarcomeres with a deletion in titin. J. Exp. Biol. doi: 10.1242/jeb.132027. [Epub ahead of print].
PubMed Abstract | CrossRef Full Text | Google Scholar
Rack, P. K. H., and Westbury, D. R. (1969). The effects of length and stimulus charge per unit on tension in the isometric cat soleus muscle. J. Physiol. 204, 443–460. doi: 10.1113/jphysiol.1969.sp008923
PubMed Abstract | CrossRef Full Text | Google Scholar
Ralston, E., Swaim, B., Czapiga, M., Hwu, Westward. L., Chien, Y. H., Pittis, M. G., et al. (2008). Detection and imaging of not-contractile inclusions and sarcomeric anomalies in skeletal muscle by second harmonic generation combined with two-photon excited fluorescence. J. Struct. Biol. 162, 500–508. doi: ten.1016/j.jsb.2008.03.010
PubMed Abstruse | CrossRef Total Text | Google Scholar
Rassier, D. Due east., and Pavlov, I. (2010). Contractile characteristics of sarcomeres arranged in series or mechanically isolated from myofibrils. Adv. Exp. Med. Biol. 682, 123–140. doi: 10.1007/978-i-4419-6366-6_7
PubMed Abstruse | CrossRef Full Text | Google Scholar
Takahashi, M., Ward, Due south. R., and Lieber, R. L. (2007). Intraoperative single-site sarcomere length measurement accurately reflects whole-musculus sarcomere length in the rabbit. J. Hand Surg. Am. 32, 612–617. doi: 10.1016/j.jhsa.2007.03.002
PubMed Abstract | CrossRef Full Text | Google Scholar
Telley, I. A., Denoth, J., Stüssi, Eastward., Pfitzer, G., and Stehle, R. (2006). Half-sarcomere dynamics in myofibrils during activation and relaxation studied by tracking fluorescent markers. Biophys. J. xc, 514–530. doi: 10.1529/biophysj.105.070334
PubMed Abstract | CrossRef Full Text | Google Scholar
Vaz, Thou. A., de la Rocha Freitas, C., Leonard, T., and Herzog, W. (2012). The force-length human relationship of the true cat soleus muscle. Muscles Ligaments Tendons J. 2, 79–84.
PubMed Abstract | Google Scholar
Walker, South. K., and Schrodt, G. R. (1974). I segment lengths and thin filament periods in skeletal muscle fibers of the rhesus monkey and the homo. Anat. Rec. 178, 63–81. doi: 10.1002/ar.10917s80107
PubMed Abstruse | CrossRef Total Text | Google Scholar
A Researcher Is Comparing The Size Of Sarcomeres In Mice To Those In Elephants. What Will He Find?,
Source: https://www.frontiersin.org/articles/10.3389/fphys.2016.00187/full
Posted by: selfancel1979.blogspot.com


0 Response to "A Researcher Is Comparing The Size Of Sarcomeres In Mice To Those In Elephants. What Will He Find?"
Post a Comment